Isothermal Amplification Protocols of Nucleic Acids Review Article
Abstract
Many contempo studies reported coronavirus point-of-care tests (POCTs) based on isothermal amplification. However, the performances of these tests have not been systematically evaluated. Cochrane Handbook for Systematic Reviews of Diagnostic Exam Accuracy was used as a guideline for conducting this systematic review. Nosotros searched peer-reviewed and preprint articles in PubMed, BioRxiv and MedRxiv up to 28 September 2020 to identify studies that provide information to calculate sensitivity, specificity and diagnostic odds ratio (DOR). Quality Cess of Diagnostic Accurateness Studies 2 (QUADAS-two) was applied for assessing quality of included studies and Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Examination Accuracy Studies (PRISMA-DTA) was followed for reporting. We included 81 studies from 65 enquiry articles on POCTs of SARS, MERS and COVID-19. Most studies had loftier risk of patient selection and index test bias but low run a risk in other domains. Diagnostic specificities were high (> 0.95) for included studies while sensitivities varied depending on type of assays and sample used. Most studies (n = 51) used reverse transcription loop-mediated isothermal distension (RT-LAMP) to diagnose coronaviruses. RT-LAMP of RNA purified from COVID-19 patient samples had pooled sensitivity at 0.94 (95% CI: 0.90–0.96). RT-LAMP of crude samples had substantially lower sensitivity at 0.78 (95% CI: 0.65–0.87). Abbott ID At present functioning was similar to RT-LAMP of rough samples. Diagnostic performances by CRISPR and RT-LAMP on purified RNA were similar. Other diagnostic platforms including RT- recombinase assisted amplification (RT-RAA) and SAMBA-II likewise offered high sensitivity (> 0.95). Future studies should focus on the apply of un-bias patient cohorts, double-blinded index examination and detection assays that do not require RNA extraction.
Introduction
Coronavirus epidemics have caused serious damage to public wellness and the global economy. Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) infected over ten k people and killed over a thousand people worldwideone. A novel coronavirus (SARS-CoV-2) that causes coronavirus affliction 2019 (COVID-19) infected over 60 million people and and so far killed over one,400,000 people (as of Nov 26th, 2020). The global Gross domestic product is predicted to shrink by nigh 1 percentage2. Rapid and depression-price diagnostic screening of a population at risk is disquisitional for controlling sources of infection. Such diagnostic adequacy also helps policy makers decide when and to what extent to ease restrictions and restore the economyiii.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) has been the gold standard for RNA virus detection4,5. Nonetheless, RT-qPCR requires upwardly to iv h sample-to-result time and needs a bulky expensive thermal cycler with fluorimetry. To fulfil the need for rapid diagnoses during disease outbreaks, point-of-intendance tests (POCTs) are needed that are cheaper, faster and deployable in the field.
Nucleic acrid detections based on isothermal amplification obviate the need for a thermal cycler thereby simplifying and speeding upward the diagnosis process. For instance, loop-mediated isothermal amplification (LAMP) relies on strand displacing DNA polymerase and primers to amplify specific DNA sequences of pathogenshalf dozen. Reverse transcription LAMP (RT-LAMP) has been practical for the detection of various RNA viruses including Ebola, Zika, Due west Nile, Influenza and Xanthous fever viruses7,eight,9,10,11. Rolling circle amplification (RCA) utilizes highly processive strand displacement DNA polymerase and circularizable oligonucleotide probes for detecting single strand Dna or RNA12. Contrary transcription insulated isothermal PCR (RT-iiPCR) relies on a temperature gradient to bulldoze denaturation/annealing/extension wheel similar to conventional PCR but in the absence of a thermal cyclerthirteen. Opposite transcription recombinase polymerase amplification (RT-RPA) or reverse transcription recombinase aided distension (RT-RAA) uses recombinase, single strand binding protein, DNA polymerase and reverse transcriptase to amplify the RNA targetxiv. Uncomplicated amplification based analysis (SAMBA) uses Deoxyribonucleic acid dependent RNA polymerase and RNA dependent DNA polymerase to alternately transcribe and contrary transcribe RNA target15. CRISPR diagnosis combines isothermal amplification techniques (such every bit RT-LAMP and RT-RPA) with specific DNA or RNA targeting ability of crRNA and Cas12 or Cas13 enzymessixteen. The outputs of these detection techniques can be coupled with fluorescent or colorimetric reporters as well as lateral catamenia strip platforms to facilitate readout processes.
While many studies presented nucleic acid POCTs for human coronaviruses, it is important to systematically evaluate and depict conclusions about the performance of POCTs and quality of these studies. These could guide clinical exercise and highlight opportunities for side by side generation POCTs. Here, we aim to determine the accurateness of nucleic acid signal-of-care diagnosis for human coronaviruses, peculiarly, SARS-CoV, MERS-CoV and SARS-CoV-two, using systematic review and meta-analysis techniques.
Methods
This study followed the guidelines in the 'Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy'17.
Eligibility criteria
Inclusion criteria
This systematic review and meta-analysis included (1) both peer-reviewed and preprint original articles on nucleic acid based POCTs; (ii) the examination must exist isothermal, i.eastward., thermal cycling is not required during the exam; (3) full text is bachelor (in any language); and (4) provide enough information to make up one's mind the number of true positive, false positive, fake negative and true negative on POCTs (performed on clinical samples) relative to a standard reference test.
Exclusion criteria
We excluded (1) studies investigating antibody examination, straight antigen tests or non-isothermal nucleic acid test, (2) studies that did not utilise clinical samples.
Search strategy
Peer-reviewed articles were searched on PubMed from its inception up to 28 September 2020 with the following search terms: (coronavirus OR COVID-nineteen OR astringent astute respiratory syndrome OR centre east respiratory syndrome) AND (rapid diagnosis OR isothermal distension). Preprint articles were searched on BioRxiv and MedRxiv from i Jan 2020 to 28 September 2020 using a search term 'isothermal amplification'. The titles, abstracts and duplicates were screened and the full text of relevant articles were reviewed by PS and cross-checked by CK. We registered our systematic review and meta-analysis on PROSPERO on Apr 21, 2020; registration number notwithstanding to exist updated.
Quality cess
The quality of each study was assessed with the Quality Cess of Diagnostic Accurateness Studies 2 (QUADAS-2)xviii. QUADAS-2 consists of 4 central domains: (one) patient pick; (two) alphabetize test; (iii) reference standard; (4) menstruation and timing. Each is assessed in terms of risk of bias and the get-go three in terms of concerns regarding applicability. These domains were assessed by using 18 signalling questions with 'yes', 'no' and 'unclear' answers. Specific criteria for what qualified as 'yeah', 'no' and 'unclear' are shown (Table S1). Then, the answers were used to gauge whether the risk of bias and the concern for the applicability of the enquiry is low, loftier or unclear. Specific criteria for each domain i.e., what qualified equally high or low risk of bias are shown (Table S2). Two reviewers (PS and CK) independently judged the quality of each study. Disagreements were resolved by consensus with boosted input from ML.
Data extraction
Data were extracted past one reviewer (PS) and where the results were unclear, the two other reviewers (CK and ML) were consulted. The parameters extracted included: citation information, types of coronaviruses, methodology, and the diagnostic accurateness of results (Tabular array S3).
Treatment articles during data extraction:
- (1)
For studies performing on different sets of samples (e.m. different patient groups from dissimilar hospitals) using the aforementioned diagnosis assays and parameter settings, we included all studies separately.
- (ii)
For studies performing on the aforementioned sets of samples using different diagnosis assays (east.k. CRISPR diagnosis vs RT-LAMP) or different variants of the aforementioned assays (due east.g. using crude samples vs purified RNA or using fluorescent readout vs lateral period strip examination), we included all studies separately. Notably, redundant samples were excluded in subgroup assay.
- (3)
For studies performing on the same set of samples using the aforementioned diagnosis assay just different parameter settings (due east.g. using different incubation times and temperatures), we included only the study that reported the highest sensitivity and specificity.
Statistical information analysis and reporting
Woods plots were generated and pooled sample statistics were calculated using R packages 'mada'19 in R plan (version 4.0.0)twenty. To avert statistical artefacts from cells containing zero values in a ii × 2 table (for example when false positive or imitation negative are zero), continuity corrections = 0.v were added to the observed frequencies when computing diagnostic odds ratio (DOR)xix. Since the sensitivity and specificity of a diagnostic test depend on each other, bivariate approaches to the meta-analysis of diagnostic accuracy was recommended for estimating sensitivity, specificity and DOR in the 'mada' package.
Since Deeks' examination is recommended for diagnostic test accurateness (DTA) meta-analyses21 formal testing for publication bias was undertaken by a regression of diagnostic log odds ratio against ane/sqrt (effective sample size), weighted by effective sample size, with P < 0.10 for the slope coefficient indicating significant asymmetry22.
The Preferred Reporting Items for a Systematic Review and Meta-assay of Diagnostic Exam Accuracy Studies' (PRISMA-DTA) was used for reporting23.
Results
Search results
We identified 2060 articles in total through database searching (Fig. 1). Afterward title and abstract screening, we excluded 1941 articles that were not principal inquiry manufactures, had no full text available or were unrelated to nucleic acid POCTs for human coronaviruses. 62 non-English manufactures were found simply only 2 met above eligibility criteria. These two articles were later excluded equally the authors did not utilise clinical samples. Later on reviewing full text, we found 65 articles with sufficient data to calculate sensitivity, specificity and diagnostic odds ratio (DOR) on clinical samples24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,sixty,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,eighty,81,82,83,84,85,86,87,88 (Table S3). Of 65 articles, 13 reported more than one study conducted on dissimilar sample groups or using different diagnostic procedures. In total, nosotros included 81 studies in our systematic review.
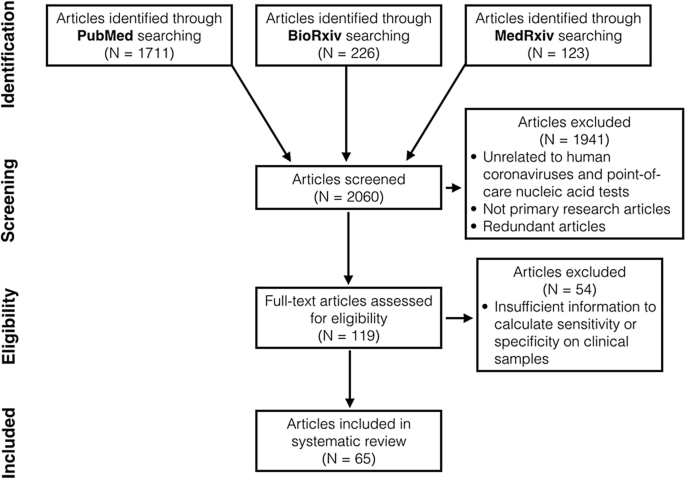
The preferred reporting items for a systematic review and meta-analysis (PRISMA) flow diagram.
Characteristics of the included studies
Nigh studies used clinical samples from Usa (n = 20 out of 81 studies), followed by Communist china (n = 17) and UK (n = 10). Most studies (n = 76) were COVID-19 diagnostic studies published or uploaded to preprint databases in 2020. Most studies (northward = 51) use RT-LAMP as nucleic acid POCTs, followed by CRISPR diagnosis (n = 12), RT-RPA/RAA (n = 7), Abbot ID At present (n = 5), and SAMBA II (n = 2). The rest were iAMP, RT-iiPCR, RT-MDCA, and RCA (n = 1 each). Over a third (n = 28) of all studies attempted to diagnose coronaviruses in crude patient samples, i.e., nasopharyngeal swabs, sputum, saliva, etc. The others (n = 53) used purified RNA from patient samples for viral diagnosis.
Quality of articles
Almost two thirds of all studies (n = 50 out of 81 studies) have high risk of patient selection bias due to not-random patient option and case–control written report design (Fig. 2, Table S2). These studies specifically recruited clinical samples known to be uninfected or infected with coronavirus. Over a tertiary of all studies have unclear gamble of patient selection bias considering these studies were not case–control only provided insufficient particular about patient inclusion/exclusion criteria. Only four studies38,49,65 has depression risk of patient pick bias.
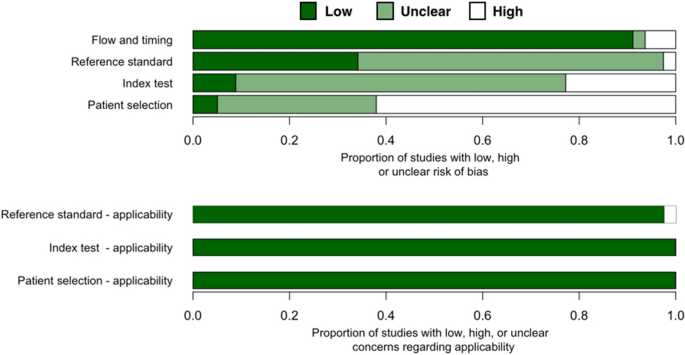
Quality assessment of diagnostic accuracy studies 2 (QUADAS-2) finding per domain for 81 studies included in this systematic review.
Over one fifth of all studies (n = 18 out of 81 studies) have loftier risk of alphabetize test bias because these tests used qualitative fluorescent or colorimetric readout without defined detection thresholds. Only seven studies had depression take chances of alphabetize test bias as these studies had quantitative detection readouts with reported thresholds. These studies also explicitly declared that their alphabetize and reference tests were washed simultaneously/in parallel to each other or that testing was blinded from each other. For other studies (n = 56), it was either unclear whether alphabetize test results were interpreted with cognition of reference test results or qualitative readout was used for interpreting the results. Thus, index examination bias of these studies are unclear.
Only 2 studies accept high gamble of reference standard bias as they used RT-PCR (non quantitative, readout result in agarose gel electrophoresis)47 or immunofluorescent analysis (IFA)69 as a reference standard test. For the residual of included studies, almost ii thirds (north = 52) take unclear run a risk of reference standard bias because these studies did not provide enough information nigh whether reference standard results were interpreted without knowledge of the results of the alphabetize test.
Most studies have a low run a risk of catamenia and timing bias with the post-obit exceptions. One study provided no information on whether the samples for a reference test (IFA) and the index exam (RT-LAMP) were taken at the same time69. Another study might have excluded some samples from the workflow87. These two studies were marked every bit having unknown hazard of flow and timing bias. Three studies were designated as having high risk because they used dissimilar standard references on different samples80, used unlike samples test flow on different sample groups65, and excluded some samples from the analysis76 (Table S2).
Our review question did non focus on any detail patient demographics. None of the included studies attempted to exclude patients based on demographics and thus had no 'business organization of patient selection applicability'(Fig. 2, Table S2). Index isothermal tests of all studies have mostly been used for POCTs and thus take low business organization of index test applicability. Reference standard tests of nearly all studies are RT-qPCR, the current gold standard for RNA virus detection. Thus, nosotros graded these studies as having low concern of standard exam applicability. Two studies that used (non-quantitative) RT-PCR47 and IFA69, were marked as having high business organization of standard exam applicability.
Sensitivity, specificity and diagnostic odd ratio (DOR) of nucleic acrid POCTs
About all studies (n = 77 out of 81) reported at least ninety% diagnosis specificity while less than two tertiary (n = 48 out of 81) reported 90% sensitivity or above (Fig. 3). Less than a third (n = fourteen out of 53) of studies that used purified RNA for diagnosis reported below xc% sensitivity (Fig. 3A). In dissimilarity, over ii thirds (n = nineteen out of 28) of studies that used crude patient samples for diagnosis reported sensitivities less than ninety% (Fig. 3B). Thus, for near studies, diagnostic specificity is of less concern than sensitivity. Moreover, diagnostic sensitivity of purified RNA is generally higher than those of crude patient samples. All studies reported DOR above one.
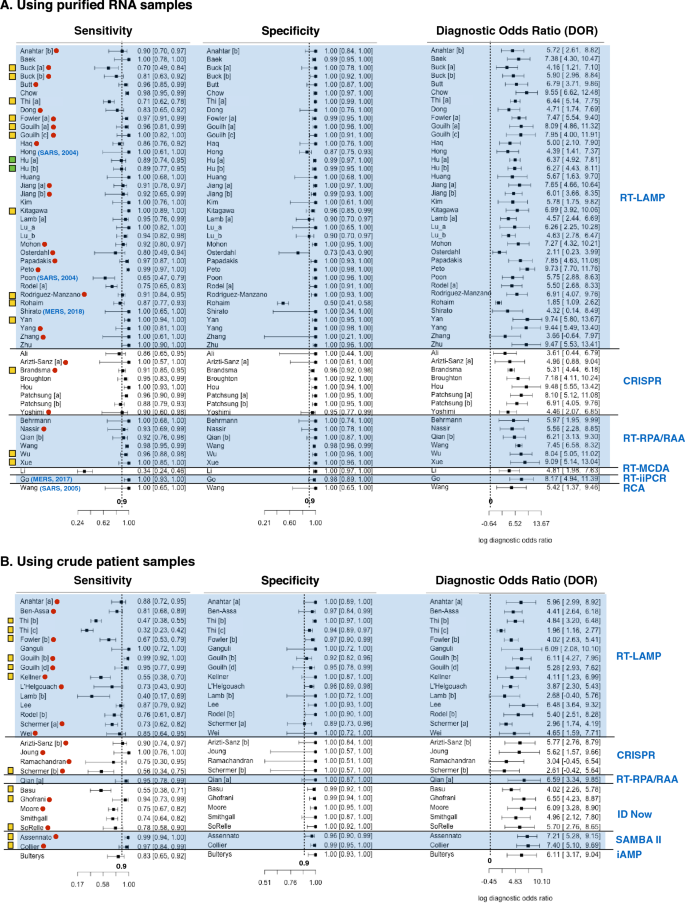
The forest plot of sensitivity, specificity and diagnostic odds ratio (DOR) of human coronavirus nucleic acid point-of-care tests (POCTs) on purified RNA samples (A) and on crude patient samples (B). Rows shows first author proper noun, and performance (sensitivity, specificity and DOR) of each study. Different studies from the aforementioned inquiry manufactures are labelled with dissimilar letter [a], [b], [c], etc. Blue parentheses after get-go author names indicates the types of coronaviruses diagnosed and publication years of the studies. All rows without blue parentheses show studies on COVID-19 diagnosis published in 2020. Red dots signal those studies that were but available as pre-prints (not peer-reviewed). The far right blueish texts point the types of diagnostic assays used in the studies. The far left yellow boxes prefacing the author names announce the studies having no QUADAS-2 domain with loftier take a chance bias or loftier business organisation of applicability but have unclear bias or concerns in some QUADAS-2 domain. Green boxes announce that the study has depression risk of bias and depression business organization of applicability in all QUADAS-ii domains. All rows without yellow or green box show studies with high chance of bias or high business concern of applicability in at least one QUADAS-2 domain.
Among studies that used RT-LAMP on purified RNA samples, the study by Rohaim et al. (2020) is conspicuously an outlier (Fig. 3A)74. This study used artificial intelligence to translate the RT-LAMP colorimetric readout. While this arroyo tin reduce assay fourth dimension and eliminate subjectivity of result interpretation, the imitation positive rate was high (approximately l% when using RT-qPCR every bit a reference exam). Osterdahl et al. (2020) is the simply study whose both sensitivity and specificity were fourscore% or below65. This report had high risk of period and timing bias considering some clinical samples were taken on different days for alphabetize test and standard reference test. Poon et al. (2004) reported the lowest sensitivity (at 65%) among all studies using RT-LAMP on purified RNA samples69. This study has a high risk of reference examination bias and high business organization of reference examination applicability because immunofluorescent analysis (IFA) was used for reference test instead of RT-qPCR. Since the antibiotic may persist much longer in patients than the virus. As a result, some samples might give a positive result to the antibody test but provide a negative result to the LAMP test. Buck et al. (2020), Thi et al. (2020) and Rodel et al. (2020) are too studies with sensitivity beneath 80%34,39,72. These studies besides reported quantity of viral RNA (as Ct value of RT-qPCR) in purified RNA sample. The authors showed that that samples with low viral RNA (i.e. high Ct value above xxx) accounted for a significant portion of coronavirus positive samples used in the studies. Since these samples were more difficult to detect (i.e. more probable to get simulated negative), this could explain apparently low sensitivities reported by these three studies.
For diagnosis of purified RNA samples using not RT-LAMP assays, all studies using RT-RPA/RAA, CRISPR diagnosis, RT-iiPCR, and RCA as alphabetize tests reported sensitivity and specificity at shut to ninety% or above (Fig. 3A). The Li et al. (2020)59 study is clearly an outlier. The study introduced a new diagnosis analysis called reverse transcription multiple cross displacement distension (RT-MCDA). The authors claimed that this new assay was more than sensitive than RT-qPCR. Nonetheless, the result showed that RT-MCDA could only detect viral RNA in 33.8% of COVID-19 confirmed patient samples. Such low sensitivity could issue from the operation of RT-MDCA itself or the fact that viral RNA in some samples was degraded as a follow-up RT-qPCR could detect COVID-19 in only thirty.7% of the same sample ready.
Nearly all studies (n = 12 out of 15) using RT-LAMP on crude patient samples reported less than 90% diagnostic sensitivity. The studies past Thi et al. (2020) and Lamb et al. (2020) reported even less than 50% sensitivity39,57. Such depression sensitivity measure could be explained by the fact that these studies used patient samples with low viral load. Excluding positive samples with Ct = thirty or higher up, the calculated sensitivities from these studies rise higher up 60% (Table S4). Diagnosis of crude patient samples using non RT-LAMP assays has sensitivity ranging from 74 to 100%, with 2 exceptions. Basu et al. (2020)29 and Schermer et al. (2020)75 reported 55% and 56% diagnostic sensitivity for ID Now and CRISPR diagnosis, respectively. For the written report by Schermer et al., all positive samples had Ct value below 30. This implies that poor sensitivity measure resulted from the performance of the analysis itself and not because the samples had low viral load. Basu et al. did non prove Ct value of samples used in their study29. Thus, it could still be possible that poor operation partially resulted from positive samples with depression viral load. In fact, another study by Smithgall et al. showed that ID Now diagnostic sensitivity is 100% for samples with Ct value not exceeding thirty simply but at 34.4% for samples with Ct value higher up thirty77 (Table S4).
Publication bias
Publication bias of all 81 included studies was determined using Deek's funnel plot test for DOR. The result indicates significant asymmetry in funnel plot (p-value = 3.203 × 10–iv).
Meta-assay of sensitivity, specificity and DOR
We performed subgroup analysis of all studies that used RT-qPCR every bit reference test and had at least 10 positive and x negative patient samples. The ii outlier studies past Rohaim et al.74 and Li et al.59 were excluded from the subgroup analysis. If multiple studies were conducted on the same set of patient samples, simply a written report with the highest sensitivity and specificity was used. For example, Patchsung et al.67 reported two CRISPR diagnosis studies on the aforementioned set up of patient samples, one using fluorescent readout and the other using lateral flow analysis. In our analysis, we included only the fluorescent readout study, which had higher sensitivity and similar specificity to the lateral period assay.
In total, 61 studies were used for subgroup analyses (Fig. 4A, Tabular array S5). These studies were divided up farther according to the types of samples used (purified RNA vs crude patient samples) and index test assays. We only estimated pooled sensitivity, specificity and diagnosis odds ratio for subgroups that had at least four studies. For the studies subgroup using RT-LAMP on purified RNA samples, nosotros also performed a further subgroup analysis to compare the performance of studies from peer-reviewed and from pre-impress articles.
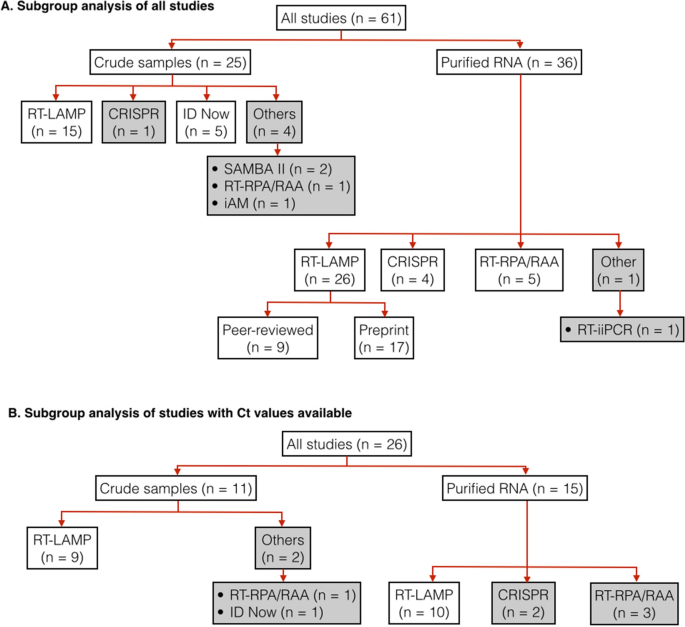
Hierarchical subgrouping of studies for meta-analysis. (A) subgrouping of all qualified studies. (B) subgrouping of only qualified studies that provide Ct values of positive samples. "n" indicates the number of studies in a subgroup. Pooled diagnosis results from subgroups in white boxes were used for calculating pooled sensitivity, specificity and DOR. Subgroups in grey boxes were non used for sensitivity, specificity, nor DOR adding.
Pooled sensitivity and specificity of all included studies are at 91% and 99%, respectively, indicating overall skilful performance of isothermal amplification based diagnosis exam and so far (Tabular array one). Pooled sensitivity of studies using purified RNA sample at 0.94 (95% CI: 0.92–0.96) is clearly higher than those using crude patient samples at 0.83 (95% CI: 0.74–0.89).
Similarly, pooled sensitivity of studies using RT-LAMP on purified RNA sample is clearly higher than that for studies using RT-LAMP on crude patient samples. Pooled sensitivity of RT-LAMP on crude samples was similar to that of ID Now. Both pooled sensitivities were lower than that of SAMBA II (Fig. three, not used in subgroup analysis). For diagnosis of purified RNA samples, pooled sensitivities of RT-LAMP, CRISPR diagnosis and RT-RPA/RAA were like.
Additionally, pooled sensitivity of RT-LAMP studies in peer-reviewed manufactures was not significantly different from that in pre-print articles.
The distribution of viral load in tested samples is one of the key factors that decide measured sensitivity of an index test. If a large fraction of positive samples used in a report have low viral load (i.eastward., high Ct value), measured sensitivity will exist low. Unfortunately, the majority of our included studies (n = 35 out of 61) do not bear witness Ct values of positive samples. Thus, it was not possible to determine the extent to which viral load in positive samples from these studies affected their measured sensitivity. For this reason, we decide to focus our analysis on only studies that reported Ct values.
In total, 26 studies were used for subgroup analyses (Fig. 4B, Table S5). Again, these studies were divided upwardly further according to types of samples used (purified RNA vs rough patient samples) and index test assays. For each subgroup analysis, nosotros calculated pooled sensitivity, specificity and DOR for the cases when all samples were used and the cases when positive samples with loftier Ct values were excluded. The 'loftier' Ct cut-off values were non the same in all included studies (depending on data available from the original inquiry articles). Most studies (n = 19 out of 26) had Ct cut-off values of xxx–33; the balance had Ct cut-off values of 34–39 (Table S4). Excluding samples with high Cts from the calculation resulted in substantial increases in sensitivity, particularly for diagnosis of crude samples (Table 1, Tabular array S4). The calculated pooled sensitivity for crude samples increases from 0.76 (95% CI: 0.57–0.88) to 0.95 (95% CI: 0.84–0.99); the calculated pooled sensitivity for RT-LAMP on crude samples increases from 0.73 (95% CI: 0.51–0.88) to 0.91 (95% CI: 0.79–0.97). Diagnostic sensitivity of purified RNA samples remained higher than those of crude patient samples. Even so, when positive samples with high Ct values were excluded, such divergence in sensitivity become smaller.
Word
To our cognition, this is the first systematic review and meta-analysis examining the performance of isothermal nucleic acid POCTs for human coronavirus. The bulk of studies that used purified RNA for diagnosis reported at least ninety% sensitivity and specificity; over a third of these studies reported 100% sensitivity and specificity. Sensitivities were generally lower for studies that used rough patient samples for diagnosis while specificities were not substantially different. Subgroup analyses confirmed the difference in sensitivity between diagnostic test performed on purified RNA and on crude patient samples. Notwithstanding, when positive samples with depression viral loads (Ct value = 30 or above) were excluded from calculation, such difference become much smaller. In other words, for samples with medium to high viral load, coronaviruses could be reliably detected directly from crude patient samples without an RNA purification step which takes more time and technical expertise.
Almost two thirds of the included studies used RT-LAMP as an index test. At the time of this writing, the simply published meta-analysis of RT-LAMP functioning was on a diagnostic accuracy of Enterovirus 71 by Lei et al. (2014)89. That meta-analysis included 907 clinical samples from 10 studies, all performed on purified RNA. Pooled data had a sensitivity of 0.99 (95% CI: 0.97–1.00), specificity of 0.97 (95% CI: 0.94–i.00) and ln(DOR) of 6.74 (95% CI: v.68–7.79). Our subgroup analysis showed that RT-LAMP on purified RNA samples had pooled sensitivity of 0.94 (95% CI: 0.90–0.96), specificity of one.00 (95% CI: 0.99–1.00) and ln(DOR) of 8.49 (95% CI: 7.06–ix.92) (Table one). Pooled sensitivity of RT-LAMP from our analysis appeared to exist lower. However, when samples with loftier Ct values were excluded, pooled sensitivity of analyses went upwards to 1.00 (95% CI: 0.89–1.00) which is within the same range as the analysis by Lei et al. Thus, our reported RT-LAMP performance is likely to reflect the true operation of this isothermal nucleic acid exam equally the functioning value is generalizable across dissimilar target viruses. This could serve equally a reference point for assessing the functioning of other diagnostic methods.
Amidst RT-LAMP studies on purified RNA, the studies past Hu et al., Kitakawa et al., Thi et al. and Yan et al.39,49,55,84 were of high quality: they tested large sample sizes and had no QUADAS-two domain with high risk of bias or business of applicability (Table S2). However, these iv studies reported contrasting results with respect to diagnostic performance. While Kitakawa et al.55 and Yan et al.84 demonstrated 100% diagnostic sensitivity, Thi et al.39 reported only seventy% sensitivity. By and large, the measured false negative rate of a diagnosis exam is loftier when the viral loads in the majority of tested samples are low.
For example, Thi et al. showed that RT-LAMP sensitivity is at 100% when the samples have viral RNA concentration equivalent to Ct of 0–25 cycles. The sensitivity decreases to well-nigh 30% at RNA concentration equivalent to Ct of 30–35 cycles and to sensitivity less than vi% at RNA concentration equivalent to Ct of 35–40 cycles. Approximately a third of positive samples in Thi et al. written report has Ct of 30–forty cycles. This could explain why RT-LAMP in this study appear to have such a high false negative rate. Yan et al. and Kitakawa et al. did not written report the distribution of viral RNA level in their tested samples. Thus, it is possible these ii studies appear to accomplish 100% sensitivity simply because nigh of their positive samples had high viral RNA level.
Viral RNA levels in samples depend on several factors including severity of the disease, timing of sample drove, types of samples and sample handling processes. Without such data, information technology is hard to determine whether the difference in observed sensitivity results from the performance of the exam itself or properties of the samples used in the test. Unfortunately, near included studies provided no information about viral RNA levels in the infected samples (equally determined by a standard reference examination, eastward.1000., RT-qPCR). Data about illness severity and sample collection timing (i.e., days afterwards affliction onset) are often missing. Future works should provide this information in order to allow a better cess of diagnosis test performance and must identify their actual limitations.
Of all included studies in this review, only two studies by Schermer et al. (2020) attempted to straight compare the coronavirus detection accurateness of CRISPR diagnosis to that of RT-LAMP using the same set up of clinical samples75. They estimated diagnostic sensitivity (in rough patient samples) of RT-LAMP at 73% and of CRISPR diagnosis at 56%. While the sensitivity of RT-LAMP shown in this study was on par with RT-LAMP performance reported by other studies in our review, diagnosis sensitivity past CRISPR was surprisingly low. Other studies including Arizti-Sanz et al. (2020) and Joung et al. (2020) estimated sensitivity of CRISPR diagnosis on rough samples at 90–100%26,52. From Schermer et al. study, CRISPR diagnosis failed to detect whatever positive samples with Ct values above 21 cycles. In contrast, the study by Joung et al. (2020) using CRISPR diagnosis could reliably detect all positive samples with Ct of 20–35 cycles52. For coronavirus detection in purified RNA samples, estimated sensitivity of RT-LAMP and of CRISPR diagnosis were most identical co-ordinate to our subgroup analysis (Table 1). Given available data, the difference in performance of CRISPR diagnosis and RT-LAMP remains inconclusive. Existing CRISPR diagnosis as well required RT-LAMP or other isothermal techniques to pre-amplify nucleic acrid targets before CRISPR detection. The utilize of cas12 or cas13 enzyme adds to the cost of CRISPR diagnosis examination kit, making it probable to be more than expensive than RT-LAMP. Future studies should straight compare and highlight the unique strength of CRISPR diagnosis relative to other isothermal techniques, for example, its ability for multiplex detection and identifying single base of operations differences in targeted genomes90,91,92.
All 7 studies using RT-RPA/RAA as an alphabetize test reported over 90% sensitivity and at least 98% specificity. Additionally, the studies by Qian et al. (2020) showed that RT-RPA/RAA based diagnosis on crude patient samples could achieve detection sensitivity level similar to diagnosis on purified RNA samples70. While nigh studies did not report Ct value of positive samples, i written report by Wu et al. (2020) demonstrated that RT-RPA/RAA based diagnosis tin can detected 91% (30 out of 33) positive samples with Ct values of thirty–36 cycles82. Together, the results from these studies suggested that RT-RPA/RAA could potentially be one of the most promising approaches for developing coronavirus POCTs. Time to come works should directly compare this assay to other nucleic acid POCTs such as RT-LAMP using the same sample sets in order to decide the bodily difference in their diagnosis functioning.
Besides RT-LAMP, CRISPR diagnosis and RT-RPA/RAA, two other diagnostic assays with over a hundred positive and negative pooled samples in our review were Abbott ID At present and SAMBA Ii. Abbott ID At present is famous for being "the fastest" (5–thirteen min) isothermal COVID-nineteen nucleic acid detection arrangement in the market. However, four out of 5 ID Now studies included in our review reported less than 80% sensitivity. Pooled data from ID Now studies have sensitivity levels on par with that of RT-LAMP applied to rough samples. Only one of these v studies by Smithgall et al. (2020) showed Ct values of positive samples77. The study reported 74% sensitivity when all samples were used for calculation. For samples with Ct beneath 30 cycles, the sensitivity is 100%. Thus, the poor performance of ID Now reported in these studies could partly outcome from having the large proportion of positive samples with low viral loads. The ii SAMBA II studies each included over a hundred tested samples and had no high gamble or concern QUADAS-two domains27,38. In these two studies, sensitivity and specificity at 97% or above were among those with highest accuracy of all the included studies in our current systematic review. Despite beingness the slowest POCTs amongst our included studies (> i h from sample to readout), SAMBA 2 is arguably one of the most promising POCTs thus far regarding diagnostic accuracy for coronavirus detection of crude patient samples. Unfortunately, neither of these ii studies reported Ct values of the samples used. Thus, given data availability for this review, nosotros still cannot directly compare the performance of this approach to other assays.
Our study identified both relevant peer-reviewed studies and preprints for deriving better scientific conclusions in diagnosis of the life-threatening novel coronavirus in a timely mode. Our report too adhered to the standard methodology of systematic review and meta-assay as indicated by the PRISMA-DTA statement93. Notwithstanding, our report had few limitations. First, about half of the included studies (n = 38 out for 81) had high risk of patient pick bias or index test bias. Such bias could pb to over-estimation of diagnosis functioning. Nonetheless, the studies that reported the highest functioning (near 100% sensitivity and 100% specificity with narrow 95% CIs) were likewise the ones with lowest QUADAS take chances and concerns in all domains27,38,41,45,55,82,83,84. Second, almost two thirds (n = 28) of included studies had non been peer-reviewed. Withal, our assay showed that sensitivity and specificity for pooled data from these preprint manuscripts was non significantly different from that of the peer-reviewed studies. Therefore, an inclusion of data from preprint manuscripts is unlikely to skew the results of our other analyses. Given that the peer-review process frequently takes at to the lowest degree a few months, the systematic review that includes preprint manuscripts is timely for guiding the management of on-going research especially during a global pandemic. Third, our assay indicated significant publication bias in included studies. Given that all nucleic acid isothermal POCTs for coronaviruses were nevertheless in an early on stage of development, about all reported diagnosis accuracy assessments were performed and published only past the same inquiry groups that developed or optimised the assays. Thus, there is probable to be bias toward reporting higher diagnosis performance. We expected that such bias would be mitigated once these POCTs are fully deployed in the field and assessed past multiple and independent research teams, not directly affiliated with POCT developers.
Nosotros identified a recent comprehensive systematic review on nucleic acid POCTs of SARS-CoV-2 by Dinnes et al. (2020)94. The review past Dinnes et al. was published later on our preprint version was bachelor online95. Dinnes et al. searched article database merely upwards to 25 May 2020 and did non include any isothermal POCT assays besides ID At present. While the systematic review by Dinnes et al. was more selective for studies that were highly relevant to ongoing clinical uses, our review offered a broader perspective on the performance of diverse competing diagnostic platforms.
In conclusion, our systematic review and meta-analysis revealed the electric current state of nucleic acrid POCTs for human coronaviruses. Overall diagnostic accuracy of these POCTs reported and then far was high but the quality of these studies was still in question. Particularly, future study should endeavour to apply united nations-bias (e.g., random or consecutive) patient cohorts and perform double-blinded index test. Such improvement in report design and methodology would enhance validity of the estimated sensitivity and specificity of POCTs. This would allow researchers and healthcare providers to brand correct decisions on which POCTs platforms to deploy or upgrade.
Sensitivity and specificity of RT-LAMP, RT-RPA/RAA and CRISPR diagnosis on purified RNA samples were not materially different. Critical information virtually viral load or factors influencing viral load was missing in near studies. Information technology is yet unclear whether CRISPR diagnosis was superior to a cheaper, simpler and more established nucleic acrid POCTs such as RT-LAMP. The performance of viral detection direct from patient samples is substantially lower than from purified RNA.SAMBA II had highest diagnostic accuracy among all POCTs for crude samples in this systematic review while Abbott ID At present had lower diagnostic accurateness. A quantum in bypassing an RNA purification step will simplify the workflow, reduce time, cost and possible errors. The improvement in these fundamental areas will bring nucleic acid POCTs toward big practical uses for surveillance of on-going and hereafter coronavirus outbreaks.
References
-
Prompetchara, E., Ketloy, C. & Palaga, T. Allowed responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 38, 1–9 (2020).
-
United Nation. https://www.un.org/sustainabledevelopment/blog/2020/04/covid-19-likely-to-shrink-global-gross domestic product-by-well-nigh-i-per-cent-in-2020/ (2020).
-
Vashist, Southward. Chiliad. In vitro diagnostic assays for COVID-19: Recent advances and emerging trends. Diagnostics 10, 202, https://doi.org/ten.3390/diagnostics10040202 (2020).
-
Centers for Illness Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. https://www.fda.gov/media/134922/download (2020).
-
Shen, M. et al. Recent advances and perspectives of nucleic acrid detection for coronavirus. J. Pharm. Anal. 10, 97–101 (2020).
-
Becherer, L. et al. Loop-mediated isothermal amplification (LAMP)-review and nomenclature of methods for sequence-specific detection. Anal. Methods. 12, 717–746 (2020).
-
Lin, X. et al. Fast and parallel detection of four ebola virus species on a microfluidic-chip-based portable reverse transcription loop-mediated isothermal amplification system. Micromachines 10 (2019).
-
da Silva, Southward. J. R., Pardee, K. & Pena, 50. Loop-mediated isothermal amplification (LAMP) for the diagnosis of Zika virus: A review. Viruses 12, 1–xx (2020).
-
Cao, Z. et al. Visual detection of West Nile virus using reverse transcription loop-mediated isothermal amplification combined with a vertical flow visualization strip. Front. Microbiol. 7, 1–9 (2016).
-
Ahn, Southward. J. et al. Rapid and uncomplicated colorimetric detection of multiple influenza viruses infecting humans using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. BMC Infect. Dis. xix, 1–12 (2019).
-
Ole Kwallah, A. et al. A existent-time reverse transcription loop-mediated isothermal amplification analysis for the rapid detection of yellowish fever virus. J. Virol. Methods 193, 23–27 (2013).
-
Goo, Northward. I. & Kim, D. Due east. Rolling circle amplification as isothermal cistron amplification in molecular diagnostics. Biochip J. x, 262–271 (2016).
-
Krishnan, M., Ugaz, V. Yard. & Burns, Grand. A. PCR in a Rayleigh-Bénard convection cell. Science 298, 793 (2002).
-
Li, J., Macdonald, J. & von Stetten, F. Review: A comprehensive summary of a decade development of the recombinase polymerase distension. Annotator 144, 31–67 (2019).
-
Lee, H. H. et al. Simple amplification-based assay: A nucleic acrid-based point-of-intendance platform for HIV-1 testing. 201, 65–72 (2010).
-
Aman, R., Mahas, A. & Mahfouz, M. Nucleic acid detection using CRISPR/Cas biosensing yechnologies. ACS Synth. Biol. 9, 1226–1233 (2020).
-
Deeks, J. J., Bossuyt, P. One thousand. & Gatsonis, C. Cochrane Handbook for Systematic Reviews of Diagnostic Examination Accuracy. (The Cochrane Collaboration, 2010).
-
Whiting, P. F. et al. QUADAS-2: A revised tool for the quality cess of diagnostic accuracy studies. Ann. Intern. Med. 154, 253–260 (2011).
-
Doebler, P & Holling, H. Meta-Assay of Diagnostic Accuracy with Mada. https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf (2020).
-
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing). https://world wide web.r-project.org/ (2020)
-
Van Enst, West. A., Ochodo, Eastward., Scholten, R. J., Hooft, L. & Leeflang, 1000. M. Investigation of publication bias in meta-analyses of diagnostic test accuracy: A meta-epidemiological study. BMC Med. Res. Methodol. fourteen, 70, https://doi.org/10.1186/1471-2288-fourteen-70 (2014).
-
Deeks, J. J., Macaskill, P. & Irwig, Fifty. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 58, 882–893 (2005).
-
McInnes, M. D. F. et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies the PRISMA-DTA argument. JAMA J. Am. Med. Assoc. 319, 388–396 (2018).
-
Ali, Z. et al. iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-two. Preprint at https://www.medrxiv.org/content/10.1101/2020.06.02.20117739v1 (2020)
-
Anahtar, Yard. N. et al. Clinical assessment and validation of a rapid and sensitive SARS-CoV-ii test using reverse-transcription loop-mediated isothermal amplification. Preprint at https://www.medrxiv.org/content/ten.1101/2020.05.12.20095638v1 (2020)
-
Arizti-Sanz, J. et al. Integrated sample inactivation, amplification, and Cas13-based detection of SARS-CoV-2. Preprint at https://www.biorxiv.org/content/10.1101/2020.05.28.119131v1 (2020)
-
Assennato, S. 1000. et al. Performance evaluation of the point-of-intendance SAMBA Ii SARS-CoV-2 Test for detection of SARS-CoV-2. Preprint at https://world wide web.medrxiv.org/content/ten.1101/2020.05.24.20100990v3 (2020)
-
Baek, Y. H. et al. Evolution of a reverse transcription-loop-mediated isothermal amplification equally a rapid early-detection method for novel SARS-CoV-two. Emerg. Microbes Infect. 9, 998–1007 (2020).
-
Basu, A. et al. Performance of abbott id at present covid-19 rapid nucleic acid distension examination using nasopharyngeal swabs transported in viral transport media and dry nasal swabs in a New York city bookish establishment. J. Clin. Microbiol. 58, e01136–20, https://doi.org/10.1128/JCM.01136-20 (2020)
-
Behrmann, O. et al. Rapid detection of SARS-CoV-ii by low volume real-fourth dimension single tube reverse transcription recombinase polymerase amplification using an exo probe with an internally linked quencher (Exo-IQ). Clin. Chem. 66, 1047–1054 (2020).
-
Ben-Assa, N. et al. SARS-CoV-two on-the-spot virus detection directly from patients. Preprint at https://www.medrxiv.org/content/10.1101/2020.04.22.20072389v2 (2020)
-
Brandsma, East. et al. Rapid, sensitive and specific SARS coronavirus-two detection : a multi-centre comparison between standard qRT-PCR and CRISPR based DETECTR. Preprint at https://world wide web.medrxiv.org/content/x.1101/2020.07.27.20147249v1 (2020)
-
Broughton, J. P. et al. CRISPR – Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 38, 870–874 (2020).
-
Buck, M. D. et al. Standard operating procedures for SARS-CoV-2 detection past a clinical diagnostic RT-LAMP assay. Preprint at https://www.medrxiv.org/content/10.1101/2020.06.29.20142430v1 (2020)
-
Bulterys, P. L. et al. Comparison of a laboratory-developed test targeting the envelope gene with three nucleic acid amplification tests for detection of SARS-CoV-2. J. Clin. Virol. 129, 104427 (2020).
-
Butt, A. One thousand., Siddique, S., An, X. & Tong, Y. Development of a dual-factor loop-mediated isothermal amplification (LAMP) detection analysis for SARS-CoV-2: A preliminary written report. Preprint at https://www.medrxiv.org/content/10.1101/2020.04.08.20056986v1 (2020)
-
Chow, F. W. N. et al. A rapid, elementary, inexpensive, and mobile colorimetric assay covid-xix-lamp for mass on-site screening of covid-19. Int. J. Mol. Sci. 21, 5380, https://doi.org/ten.3390/ijms21155380 (2020).
-
Collier, D. A. et al. Rapid point of care nucleic acid testing for SARS-CoV-two in hospitalised patients: A clinical trial and implementation study. Preprint at https://world wide web.medrxiv.org/content/x.1101/2020.05.31.20114520v2 (2020).
-
Thi, V. L. D. et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-two RNA in clinical samples. Sci. Transl. Med. 12, eabc7075, https://doi.org/10.1126/scitranslmed.abc7075 (2020).
-
Dong, Y. et al. Comparative evaluation of 19 reverse transcription loop-mediated isothermal distension assays for detection of SARS-CoV-2. Preprint at https://www.medrxiv.org/content/10.1101/2020.07.22.20159525v1 (2020)
-
Fowler, V. L. et al. A highly effective reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid detection of SARS-CoV-2. Preprint at infection https://www.medrxiv.org/content/x.1101/2020.06.thirty.20142935v4 (2020).
-
Ganguli, A. et al. Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 117, 22727–22735 (2020).
-
Ghofrani, M. et al. Performance characteristics of the ID Now COVID-19 assay : A regional wellness care system feel. Preprint at https://world wide web.medrxiv.org/content/10.1101/2020.06.03.20116327v1 (2020)
-
Go, Y. Y. et al. Evaluation and clinical validation of two field-deployable reverse transcription-insulated isothermal PCR assays for the detection of the middle east respiratory syndrome-coronavirus. J. Mol. Diagn. 19, 817–827 (2017).
-
Gouilh, One thousand. A. R. et al. An easy, reliable and rapid SARS-CoV2 RT-LAMP based test for point-of-intendance and diagnostic lab. Preprint at https://world wide web.medrxiv.org/content/ten.1101/2020.09.25.20200956v1 (2020)
-
Haq, F. et al. Development Optimization and Validation of RT-LAMP based COVID-xix Facility in Pakistan. Preprint at https://www.biorxiv.org/content/10.1101/2020.05.29.124123v1 (2020).
-
Hong, T. C. et al. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus evolution and evaluation of a novel loop-mediated isothermal amplification method for rapid detection. J. Clin. Microbiol. 42, 1956–1961 (2004).
-
Hou, T. et al. Development and evaluation of a rapid CRISPR-based diagnostic for COVID-19. PLoS Pathog. 16, e1008705, https://doi.org/10.1371/journal.ppat.1008705 (2020).
-
Hu, X. et al. Development and clinical awarding of rapid and sensitive loop-mediated isothermal amplification test for SARS-CoV-2 infection. mSphere 5, e00808–20, https://doi.org/x.1128/mSphere.00808-20 (2020).
-
Huang, Westward. E. et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 13, 950–961 (2020).
-
Jiang, Grand. et al. Development and validation of a rapid unmarried-footstep reverse transcriptase loop-mediated isothermal distension (RT-LAMP) system potentially to exist used for reliable and high-throughput screening of COVID-19. Preprint at https://www.medrxiv.org/content/10.1101/2020.03.15.20036376v2 (2020).
-
Joung, J. et al. Point-of-care testing for COVID-xix using SHERLOCK diagnostics. Preprint at https://www.medrxiv.org/content/10.1101/2020.05.04.20091231v1 (2020).
-
Kellner, M. J. et al. A rapid, highly sensitive and open-access SARS-CoV-ii detection assay for laboratory and domicile testing. https://world wide web.biorxiv.org/content/x.1101/2020.06.23.166397v2 (2020).
-
Kim, Y. et al. Single-strand RPA for rapid and sensitive detection of SARS-CoV-2 RNA. Preprint at https://www.medrxiv.org/content/x.1101/2020.08.17.20177006v2 (2020).
-
Kitagawa, Y. et al. Evaluation of rapid diagnosis of novel coronavirus affliction (COVID-19) using loop-mediated isothermal distension. J. Clin. Virol. 129, 104446, https://doi.org/ten.1016/j.jcv.2020.104446 (2020).
-
L'Helgouach, Northward. et al. EasyCOV : LAMP based rapid detection of SARS-CoV-2 in saliva. Preprint at https://www.medrxiv.org/content/10.1101/2020.05.30.20117291v1 (2020).
-
Lamb, L. E., Bartolone, S. N., Ward, E. & Chancellor, M. B. Rapid detection of novel coronavirus/severe acute respiratory syndrome coronavirus 2 mediated isothermal distension. PLoS One fifteen, e0234682, https://doi.org/10.1371/periodical.pone.0234682 (2020).
-
Lee, J. Y. H. et al. Validation of a single-pace, single-tube reverse transcription loop-mediated isothermal amplification analysis for rapid detection of SARS-CoV-ii RNA. J. Med. Microbiol. 69, 1169–1178 (2020).
-
Li, S. et al. Highly sensitive and specific diagnosis of coronavirus disease 19 (COVID-19) by reverse transcription multiple cross deportation amplification-labelled nanoparticles biosensor. Eur. Respir. J. 19, 2002060, https://doi.org/10.1183/13993003.02060-2020 (2020).
-
Lu, R. et al. A novel contrary transcription loop-mediated isothermal amplification method for rapid detection of sars-cov-two. Int. J. Mol. Sci. 21, 2826, https://doi.org/x.3390/ijms21082826. (2020).
-
Lu, R. et al. Development of a novel opposite transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-ii. Virol. Sin. 35, 344–347 (2020).
-
Mohon, A. N. et al. Development and validation of direct RT-LAMP for SARS-CoV-2. Preprint at https://world wide web.medrxiv.org/content/10.1101/2020.04.29.20075747v2 (2020).
-
Moore, N. 1000., Li, H., Schejbal, D., Lindsley, J. & Hayden, K. Yard. Comparison of two commercial molecular tests and a laboratory-developed modification of the CDC 2019-nCOV RT-PCR assay for the qualitative detection of SARS-CoV-2 from upper respiratory tract specimens. Preprint at https://www.medrxiv.org/content/10.1101/2020.05.02.20088740v1 (2020).
-
Nassir, A. A., Baptiste, M. J., Mwikarago, I. & Habimana, M. R. RPA-Based method for the detection of SARS-CoV-two. Preprint at https://www.medrxiv.org/content/ten.1101/2020.09.17.20196402v1 (2020).
-
Osterdahl, M. et al. Detecting SARS-CoV-ii at Point of Care: Preliminary Data Comparing Loop-Mediated Isothermal Distension (LAMP) to PCR. Preprint at https://world wide web.medrxiv.org/content/10.1101/2020.04.01.20047357v1 (2020).
-
Papadakis, G. et al. Real-time colorimetric LAMP methodology for quantitative nucleic acids detection at the point-of-care. Preprint at https://world wide web.biorxiv.org/content/10.1101/2020.07.22.215251v1 (2020).
-
Patchsung, M. et al. Clinical validation of a Cas13-based assay for the detection of SARS-CoV-two RNA. Nat. Biomed. Eng. https://doi.org/x.1038/s41551-020-00603-x (2020).
-
Peto, L. et al. Diagnosis of SARS-CoV-two infection with LamPORE, a loftier- throughput platform combining loop-mediated isothermal distension and nanopore sequencing. Preprint at https://world wide web.medrxiv.org/content/x.1101/2020.09.18.20195370v1 (2020).
-
Poon, 50. L. M. et al. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus past a loop-mediated isothermal amplification assay. Clin. Chem. 50, 1050–1052 (2004).
-
Qian, J. et al. An enhanced isothermal amplification assay for viral detection. Preprint at https://www.biorxiv.org/content/ten.1101/2020.05.28.118059v1 (2020).
-
Ramachandran, A. et al. Electric-field-driven microfluidics for rapid CRISPR-based diagnostics and its application to detection of SARS-CoV-2. Preprint at https://www.biorxiv.org/content/10.1101/2020.05.21.109637v1 (2020).
-
Rodel, J. et al. Use of the Veriplex SARS-CoV-2 RT-LAMP equally a rapid molecular assay to complement RT-PCR for COVID-xix diagnosis. J. Clin. Virol. 132, 104616, https://doi.org/10.1016/j.jcv.2020.104616 (2020).
-
Rodriguez-manzano, J. et al. A handheld betoken-of-care system for rapid detection of SARS-CoV-2 in nether 20 minutes. Preprint at https://www.medrxiv.org/content/ten.1101/2020.06.29.20142349v1 (2020).
-
Rohaim, M. A. et al. Artificial intelligence-assisted loop mediated isothermal amplification (AI-LAMP) for rapid detection of SARS-CoV-2. Viruses 12, 972, https://doi.org/ten.3390/v12090972 (2020).
-
Schermer, B. et al. Rapid SARS-CoV-ii testing in main textile based on a novel multiplex LAMP assay. Preprint at https://www.medrxiv.org/content/10.1101/2020.06.eighteen.20130377v1 (2020).
-
Shirato, K. et al. Evolution of fluorescent reverse transcription loop-mediated isothermal amplification (RT-LAMP) using quenching probes for the detection of the Middle E respiratory syndrome coronavirus. J. Virol. Methods 258, 41–48 (2018).
-
Smithgall, M. C., Scherberkova, I., Whittier, Southward. & Light-green, D. A. Comparison of cepheid Xpert Xpress and Abbott ID now to Roche cobas for the rapid detection of SARS-CoV-2. J. Clin. Virol. 128, 104428 (2020).
-
SoRelle, J. et al. Evaluation of symptomatic patient saliva as a sample type for the Abbott ID NOW COVID-nineteen assay. Preprint at https://world wide web.medrxiv.org/content/ten.1101/2020.06.01.20119198v1 (2020).
-
Wang, B. et al. Rapid and sensitive detection of severe acute respiratory syndrome coronavirus by rolling circle amplification. J. Clin. Microbiol. 43, 2339–2344 (2005).
-
Wang, J. et al. Multiple-center clinical evaluation of an ultrafast unmarried-tube assay for SARS-CoV-two RNA. Clin. Microbiol. Infect. 26, 1076–1081 (2020).
-
Wei, S. et al. Straight diagnostic testing of SARS-CoV-2 without the need for prior RNA extraction. Preprint at https://www.medrxiv.org/content/ten.1101/2020.05.28.20115220v1 (2020).
-
Wu, T. et al. A contrary-transcription recombinase-aided amplification assay for the rapid detection of North cistron of severe acute respiratory syndrome coronavirus two(SARS-CoV-2). Virology 549, 1–iv (2020).
-
Xue, G. et al. Reverse-transcription recombinase-aided amplification analysis for rapid detection of the 2019 novel coronavirus (SARS-CoV-2). Anal. Chem. 92, 9699–9705 (2020).
-
Yan, C. et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) past a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 26, 773–779 (2020).
-
Yang, W. et al. Rapid Detection of SARS-CoV-two Using Contrary transcription RT-LAMP method. Preprint at https://www.medrxiv.org/content/10.1101/2020.03.02.20030130v2 (2020).
-
Yoshimi, 1000. et al. Rapid and accurate detection of novel coronavirus SARS-CoV-2 using CRISPR-Cas3. Preprint at https://www.medrxiv.org/content/10.1101/2020.06.02.20119875v1 (2020).
-
Zhang, Y. et al. Rapid Molecular Detection of SARS-CoV-2 (COVID-19) Virus RNA Using Colorimetric LAMP. Preprint at https://www.medrxiv.org/content/x.1101/2020.02.26.20028373v1 (2020).
-
Zhu, X. et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-nineteen. Biosens. Bioelectron. 166, 112437, https://doi.org/x.1016/j.bios.2020.112437 (2020).
-
Lei, 10., Wen, H., Zhao, Fifty. & Yu, X. Performance of reversed transcription loop-mediated isothermal distension technique detecting EV71: A systematic review with meta-analysis. Biosci. Trends 8, 75–83 (2014).
-
Lee, Due south. H., Park, Y. H., Jin, Y. B., Kim, S. U. & Hur, J. Thou. CRISPR diagnosis and therapeutics with single base of operations pair precision. Trends Mol. Med. 26, 337–350 (2020).
-
Gootenberg, J. S. et al. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a and Csm6. Science 360, 439–444 (2018).
-
Pardee, K. et al. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 165, 1255–1266 (2016).
-
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6 (2009).
-
Dinnes, J. et al. Rapid, betoken-of-intendance antigen and molecular-based tests for diagnosis of SARS-CoV-ii infection (Review). Cochrane Database Syst. Rev. 8, CD013705, https://doi.org/10.1002/14651858.CD013705 (2020).
-
Subsoontorn, P., Lohitnavy, One thousand. & Kongkaew, C. The diagnostic accuracy of nucleic acid point-of-care tests for human coronavirus: A systematic review and meta-analysis. Preprint at https://www.medrxiv.org/content/10.1101/2020.07.09.20150235v1 (2020).
Acknowledgements
Nosotros would like to give thanks Dr. Norman Scholfield, Kinesthesia of Pharmaceutical Sciences, Naresuan University, for editing the manuscript.
Funding
We received no financial support from any individual or system.
Author information
Affiliations
Contributions
P.S. conceptualized the enquiry. P.South. and C.1000. performed systematic review and meta-analysis. P.S., C.1000. and M.50. wrote the manuscript.
Corresponding writer
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher'due south annotation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open up Access This article is licensed under a Creative Eatables Attribution 4.0 International License, which permits employ, sharing, accommodation, distribution and reproduction in any medium or format, as long as you requite appropriate credit to the original writer(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were fabricated. The images or other third party fabric in this commodity are included in the article's Creative Eatables licence, unless indicated otherwise in a credit line to the material. If cloth is not included in the commodity's Creative Commons licence and your intended utilize is non permitted by statutory regulation or exceeds the permitted use, y'all will need to obtain permission directly from the copyright holder. To view a re-create of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Subsoontorn, P., Lohitnavy, M. & Kongkaew, C. The diagnostic accurateness of isothermal nucleic acid point-of-care tests for human being coronaviruses: A systematic review and meta-analysis. Sci Rep x, 22349 (2020). https://doi.org/ten.1038/s41598-020-79237-7
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-020-79237-7
Further reading
Comments
Past submitting a annotate you hold to abide past our Terms and Community Guidelines. If yous detect something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Source: https://www.nature.com/articles/s41598-020-79237-7
Post a Comment for "Isothermal Amplification Protocols of Nucleic Acids Review Article"